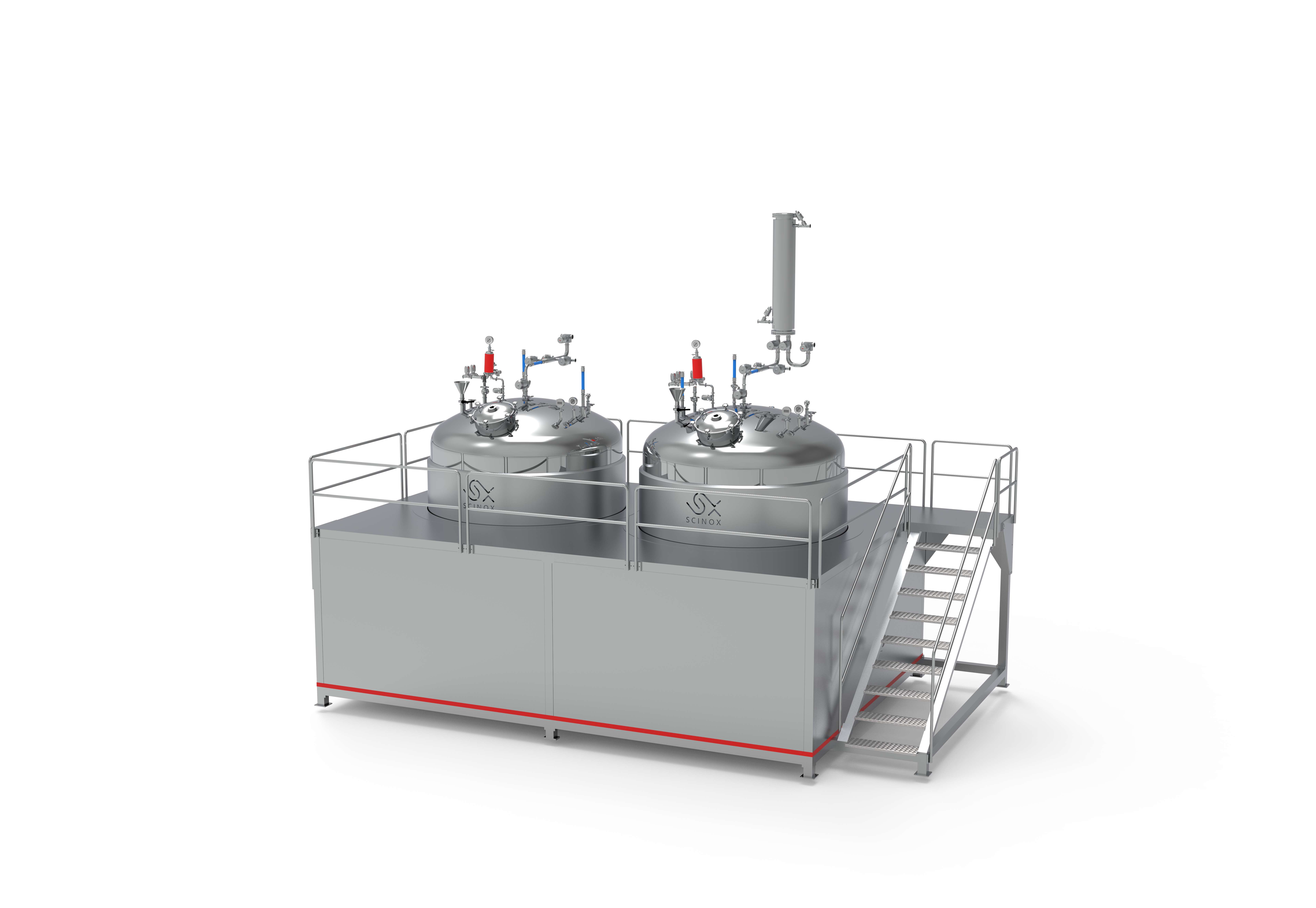
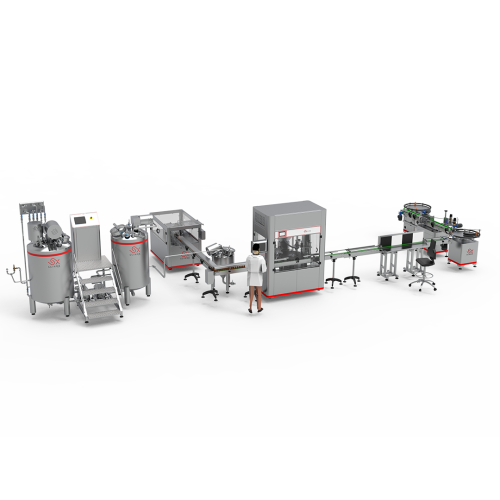
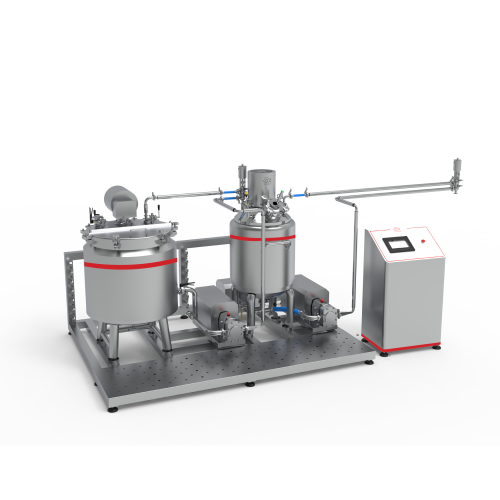
Description
• Easy investment through:
– The ability to switch from one product to another within a short time.
– Cleaning in place system CIP.
– Sterilization in place system SIP.
– Flexibility in calibrating and controlling all work parameters.
– Outstanding performance, easy to use and maintain.
• The equipment is highly efficient, flexible, and has low energy consumption.
• The necessary Documents such as installation, operation and maintenance manual.